Panthera is an experienced clinical research company which helps develop future treatments for medical conditions.
The Chorley Surgery is now working with Panthera on trials in relation to -Chronic Obstructive Pulmonary Disease (COPD) and Chronic Bronchitis, and Multiple Sclerosis
Outlined below you can find further information on both of the above named trials. Additional details on patient and data journey is also explained
Got a question about research and clinical trials? Please scroll to the bottom of the page for Frequently Asked Questions and answers


Questions people often ask about research and clinical trials
Q1 Why am I getting letters about taking part in research?
A1 Your practice takes part in Panthera research as they feel it is the right of every patient to have access to the latest treatments and clinical trials for new treatments. Some of these treatments are only in the research phase and are not routinely available on the NHS. It’s one of the ways the NHS is constantly striving to improve its standard of care.
Q2 Do I have to take part in Research and if I don’t will it affect my care?
A2 No you do not have to take part in Panthera research and if you decide not to take part it will not affect the quality of your care or your relationship with your GP or the practice. You can opt out of any single trial and you can opt out of all research if that is best for you. If you would like to opt out of a single trial you have received a letter about or for all future research, please let your practice know and they will ensure that you are not contacted again.
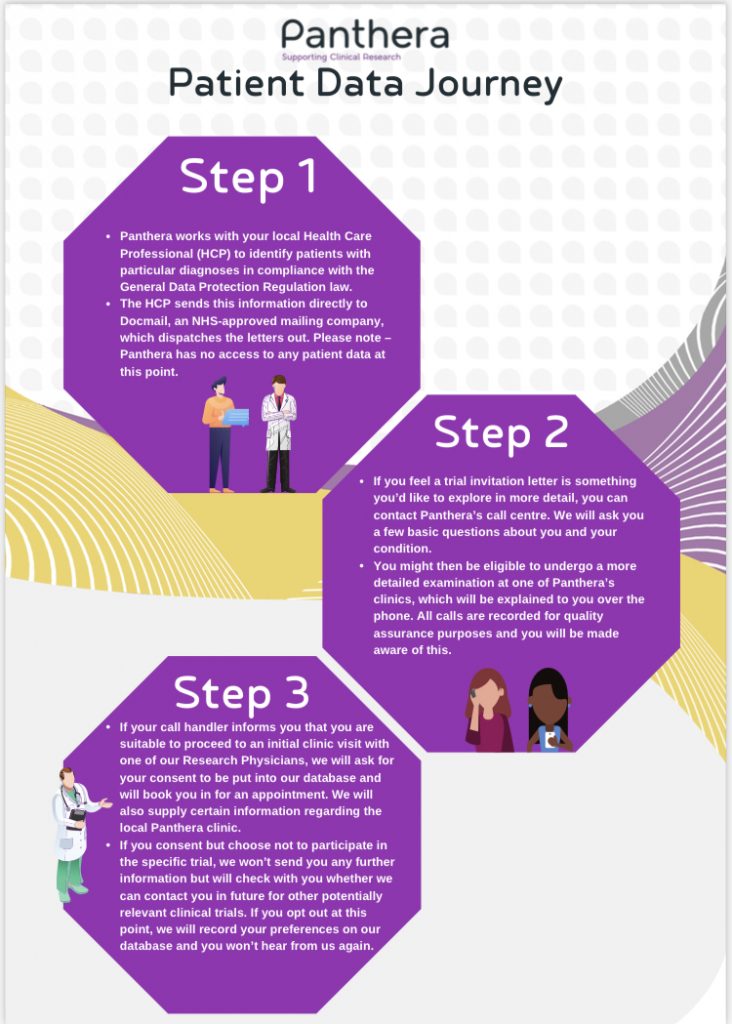
Q3 Does my practice give or sell my personal data to other research companies?
A3 No – Research studies are run by Panthera because they want to improve treatments for a range of people, with many different medical conditions. We do not share or sell your personal data and please be assured that no personal information leaves your practice, without consent.
Panthera does not know who has been sent a letter unless you decide to take part and contact them.
Q4 I have been sent a letter about a research study that says I have ‘osteoarthritis’ or other condition, I did not know I had. Why has this happened?
A4 All medical conditions are given a ‘code’ on your GP practice database. Your practice identifies patients which match the code of the research area of the clinical trial they are participating in. Patients should already know that they have a particular condition, for example Diabetes or Angina but occasionally, a diagnosis code may be in your records from a hospital letter or X-ray result and you may either not be aware of the medical name for the condition or think of it under another name. An example may be Dermatitis – this is also known as eczema or contact dermatitis or atopic dermatitis.
Q5 I have rung my practice and the reception staff seemed to have no idea about the research invitation
A5 Some Panthera research studies are run centrally and letters are sent out from the admin team at your GP clinic. We try our best to ensure that all our clinical and reception staff are aware of letters and the latest trials but the practice may be very busy, and so staff may be unaware of new updates. We are trying to change this by sharing this Q&A page within the clinical team.
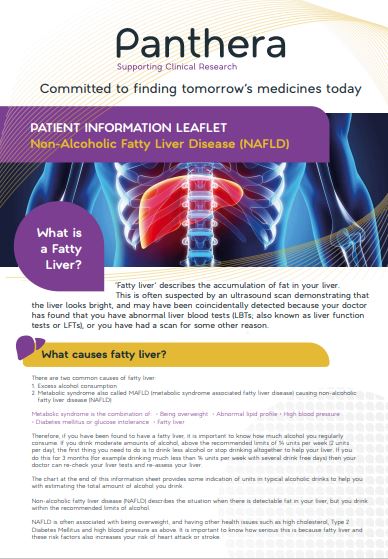
Q6 How does participation in a clinical trial work?
A6 A clinical trial is a way of measuring the effectiveness of new medicines and
treatments.
There are 4 steps to taking part in a clinical trial:
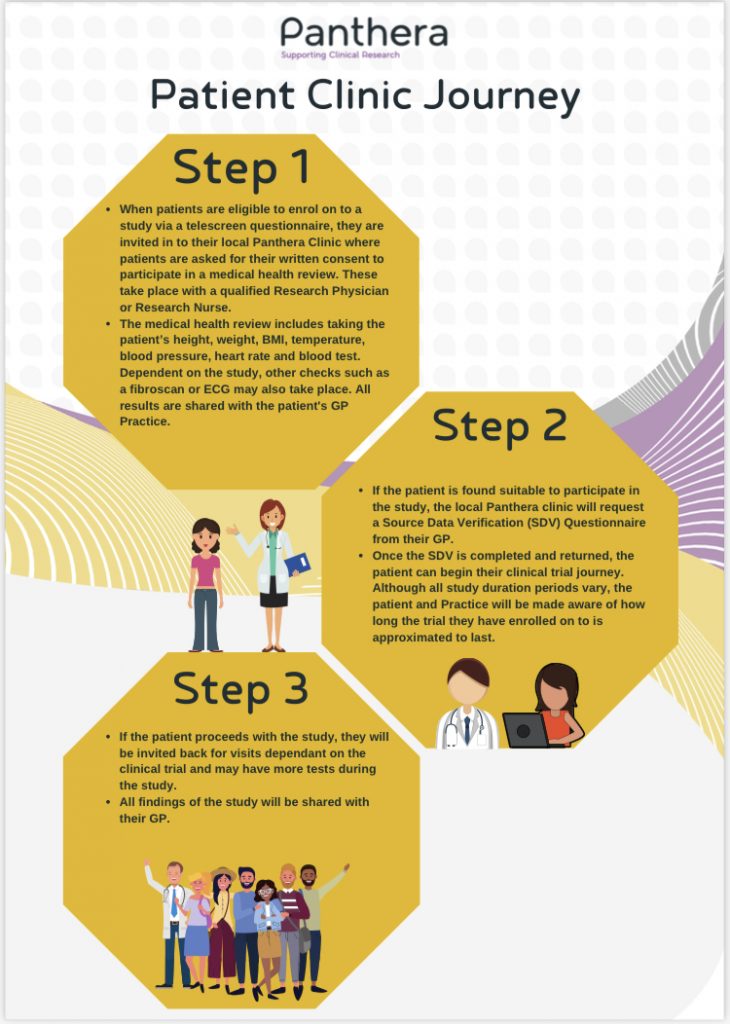
i. Register your interest: when you contact Panthera (details will be on your letter), they will ask you some questions to see if you are suitable for one of their trials. These questions are quite thorough because it is important that they learn more about you so they can find the right trial for your requirements.
If you are eligible, you’ll be invited to attend your local Panthera Clinic, where you will meet our clinical staff and be asked some further questions about your health to establish if you are eligible for the clinical trial. You will also be told of the potential benefits and risk factors and provided with an Informed Consent Form.
ii. Consent: you will be provided with a consent form, which explains all aspects of the trial and its potential impact. You should review this form thoroughly and discuss with your family and friends, before making a decision.
iii. Starting your Treatment Plan: If you decide to take part in a trial, you will be given a simple clinical trial plan to follow – this may include new medicines or treatments.
Your condition will be monitored throughout the trial and you will be offered specialist advice and support to help guide you through the process.
iv. Continuing your Treatment Plan: Your clinical trial plan may require you to visit the local Research clinic regularly – this will not be your usual GP clinic.
Panthera Limited will help with your travel arrangements, to ensure your journeys are as quick and as painless as possible.
If you decide that you would like to take part in the clinical trial please note that you can withdraw from the clinical trial at any time.
Q7 Why should I take part in a clinical trial?
A7 People choose to participate in clinical trials for different reasons. Some want to play a more active role in their own health care. Others may want to help researchers achieve a medical breakthrough, or find better treatments for various conditions. After all, everything from aspirin to chemotherapy has been through a clinical trial before being prescribed by your doctor.
Whatever the motivation, when you choose to join a clinical trial, you will be helping future generations to lead healthier lives.
In addition, all patients receive:
• Specialist advice and support throughout the study
• Help with transport to and from our clinics or reimbursement of reasonable travel
expenses
• Complimentary routine health checks